Imaging the 3D structure of a single virus
Using the intense beam of the world’s most powerful x-ray free-electron laser (XFEL)
March 2, 2015
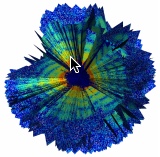
First three-dimensional reconstruction of the inside of the giant mimivirus particle, using an X-ray free-electron laser (credit: Tomas Ekeberg et al./Physical Review Letters)
By measuring a series of diffraction pattern from a virus injected into an XFEL beam, researchers at Stanford’s Linac Coherent Light Source (LCLS) have determined the first three-dimensional structure of a virus, using a mimivirus.
X-ray crystallography has solved the vast majority of the structures of proteins and other biomolecules. The success of the method relies on growing large crystals of the molecules, which isn’t possible for all molecules.
“Free-electron lasers provide femtosecond X-ray pulses with a peak brilliance ten billion times higher than any previously available X-ray source,” the researchers note in a paper inPhysical Review Letters. “Such a large jump in one physical quantity is very rare, and can have far reaching implications for several areas of science. It has been suggested that such pulses could outrun key damage processes and allow structure determination without the need for crystallization.”
The current resolution of the technique (about 100 nanometers) would be sufficient to image important pathogenic viruses like HIV, influenza and herpes, and further improvements may soon allow researchers to tackle the study of single proteins, the scientists say.
Mimivirus is one of the largest known viruses. The viral capsid is about 450 nanometers in diameter and is covered by a layer of thin fibres. A 3D structure of the viral capsid exists, but the 3D structure of the inside was previously unknown.
Abstract for Three-dimensional reconstruction of the giant mimivirus particle with an x-ray free-electron laser
We present a proof-of-concept three-dimensional reconstruction of the giant Mimivirus particle from experimentally measured diffraction patterns from an X-ray free-electron laser. Three-dimensional imaging requires the assembly of many two-dimensional patterns into an internally consistent Fourier volume. Since each particle is randomly oriented when exposed to the X-ray pulse, relative orientations have to be retrieved from the diffraction data alone. We achieve this with a modified version of the expand, maximize and compress (EMC) algorithm and validate our result using new methods.
No comments:
Post a Comment