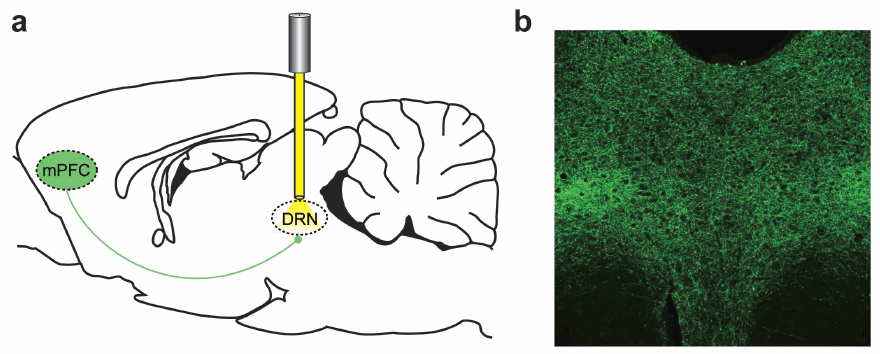
Effects of stimulating the medial prefrontal cortex (mPFC) (a) Optogenetic inhibition of nerve axons in the dorsal raphe nucleus (DRN), implicated in major depressive disorder (b) Fluorescence in the DRN detects effects. (Credit: Melissa R. Warden et al./Nature)
Karl Deisseroth, MD, PhD, a professor of bioengineering and of psychiatry and behavioral sciences at Stanford University, and postdoctoral scholar Melissa Warden, PhD, have isolated the neurons that carry the split-second decisions to act, from the higher brain to the brain stem. In doing so, they have provided insight into the causes of severe brain disorders such as depression.
In organisms as complex as humans, the neural mechanisms that help answer the question, “Is it worth my effort?” can fail, leading to debilitating mental illnesses. Major depressive disorder, for instance, which affects nearly 20 percent of people at some point in life, is correlated with underperformance in the parts of the brain involved in motivation. But researchers have struggled to work out the exact cause and effect.
“It’s challenging because we do not have a fundamental understanding of the circuitry that controls this sort of behavioral pattern selection,” Deisseroth said. ”We don’t understand what the brain is doing wrong when these behaviors become dysfunctional, or even what the brain is supposed to be doing when things are working right. This is the level of the mystery we face in this field.”
Clinicians refer to this slowing down of motivation in depressed patients as “psychomotor retardation.” According to Deisseroth, who is also a practicing psychiatrist, patients may experience this symptom mentally, finding it hard to envision the positive results of an action, or, he said, they may feel physically heavy, like their limbs just do not want to move.
“This is one of the most debilitating aspects of depression, and motivation to take action is something that we can model in animals. That’s the exciting opportunity for us as researchers,” said Deisseroth, who also holds the D.H. Chen Professorship.
Light coercion
Psychiatrists, Deisseroth included, believe the will to act may be born in the prefrontal cortex — the foremost part of the brain that helps plan and coordinate action. It then zips through the brain as a series of electrical signals, passing from neuron to neuron along countless branching pathways until it reaches the nerves that directly implement movement. Until this study, however, it was not clear which of these pathways might control the willingness to meet challenges, or the anticipation that action might be worthwhile in a difficult situation.
To isolate these pathways relevant to depression, Deisseroth’s team needed to stimulate specific brain cells in rodents and observe changes in their behavior. They used optogenetics, a technique Deisseroth developed at Stanford in 2005, which has since revolutionized the fields of bioengineering and neuroscience.
Green algae produce a protein called channelrhodopsin that makes them sensitive to sunlight. Borrowing and engineering the gene for this protein, Deisseroth has been able to create neurons that respond to light delivered from fiber-optic cables. He can turn the neurons on and off by sending bursts of light to activate different areas of the brain and then observe the effects on behavior.
Working backward
Surprisingly, the researchers found that simply stimulating the prefrontal cortices of rodents didn’t motivate them to try any harder in a laboratory challenge. It turns out that motivation is not as simple as stimulating a region of the brain. Instead of one switch in the prefrontal cortex that turns motivation on, multiple switches work in concert. Some neurons excite motivated activity and others inhibit it. Broadly stimulating the executive part of the brain will not generate a simple effect on behavior.
“It’s one step more subtle,” said Deisseroth, “but this is something that optogenetics was very well-suited to resolve.”
An optogenetic method called projection targeting allowed the scientists to work backward from the brain stem and find the exact pathway from neurons in the prefrontal cortex that signal motivation.
The researchers first introduced their light-sensitive protein into cells in the prefrontal cortex. The light sensitivity then spread out like the branches of a tree through all the outgoing connections and eventually made its way to the brain stem, making those regions light sensitive, too.
Then, illuminating the newly light-sensitive regions of the brain stem thought to control motivational movement, Deisseroth and Warden watched the behavioral effects as a subgroup of neurons in the prefrontal cortex that sent connections to the brain stem were activated. They could see not only which cells are possibly involved in motivation, but the way motivation moves from one brain region to another./.../

Effects of stimulating the medial prefrontal cortex (mPFC) (a) Optogenetic inhibition of nerve axons in the dorsal raphe nucleus (DRN), implicated in major depressive disorder (b) Fluorescence in the DRN detects effects. (Credit: Melissa R. Warden et al./Nature)
Karl Deisseroth, MD, PhD, a professor of bioengineering and of psychiatry and behavioral sciences at Stanford University, and postdoctoral scholar Melissa Warden, PhD, have isolated the neurons that carry the split-second decisions to act, from the higher brain to the brain stem. In doing so, they have provided insight into the causes of severe brain disorders such as depression.
In organisms as complex as humans, the neural mechanisms that help answer the question, “Is it worth my effort?” can fail, leading to debilitating mental illnesses. Major depressive disorder, for instance, which affects nearly 20 percent of people at some point in life, is correlated with underperformance in the parts of the brain involved in motivation. But researchers have struggled to work out the exact cause and effect.
“It’s challenging because we do not have a fundamental understanding of the circuitry that controls this sort of behavioral pattern selection,” Deisseroth said. ”We don’t understand what the brain is doing wrong when these behaviors become dysfunctional, or even what the brain is supposed to be doing when things are working right. This is the level of the mystery we face in this field.”
Clinicians refer to this slowing down of motivation in depressed patients as “psychomotor retardation.” According to Deisseroth, who is also a practicing psychiatrist, patients may experience this symptom mentally, finding it hard to envision the positive results of an action, or, he said, they may feel physically heavy, like their limbs just do not want to move.
“This is one of the most debilitating aspects of depression, and motivation to take action is something that we can model in animals. That’s the exciting opportunity for us as researchers,” said Deisseroth, who also holds the D.H. Chen Professorship.
Light coercion
Psychiatrists, Deisseroth included, believe the will to act may be born in the prefrontal cortex — the foremost part of the brain that helps plan and coordinate action. It then zips through the brain as a series of electrical signals, passing from neuron to neuron along countless branching pathways until it reaches the nerves that directly implement movement. Until this study, however, it was not clear which of these pathways might control the willingness to meet challenges, or the anticipation that action might be worthwhile in a difficult situation.
To isolate these pathways relevant to depression, Deisseroth’s team needed to stimulate specific brain cells in rodents and observe changes in their behavior. They used optogenetics, a technique Deisseroth developed at Stanford in 2005, which has since revolutionized the fields of bioengineering and neuroscience.
Green algae produce a protein called channelrhodopsin that makes them sensitive to sunlight. Borrowing and engineering the gene for this protein, Deisseroth has been able to create neurons that respond to light delivered from fiber-optic cables. He can turn the neurons on and off by sending bursts of light to activate different areas of the brain and then observe the effects on behavior.
Working backward
Surprisingly, the researchers found that simply stimulating the prefrontal cortices of rodents didn’t motivate them to try any harder in a laboratory challenge. It turns out that motivation is not as simple as stimulating a region of the brain. Instead of one switch in the prefrontal cortex that turns motivation on, multiple switches work in concert. Some neurons excite motivated activity and others inhibit it. Broadly stimulating the executive part of the brain will not generate a simple effect on behavior.
“It’s one step more subtle,” said Deisseroth, “but this is something that optogenetics was very well-suited to resolve.”
An optogenetic method called projection targeting allowed the scientists to work backward from the brain stem and find the exact pathway from neurons in the prefrontal cortex that signal motivation.
The researchers first introduced their light-sensitive protein into cells in the prefrontal cortex. The light sensitivity then spread out like the branches of a tree through all the outgoing connections and eventually made its way to the brain stem, making those regions light sensitive, too.
Then, illuminating the newly light-sensitive regions of the brain stem thought to control motivational movement, Deisseroth and Warden watched the behavioral effects as a subgroup of neurons in the prefrontal cortex that sent connections to the brain stem were activated. They could see not only which cells are possibly involved in motivation, but the way motivation moves from one brain region to another./.../
A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge
- Nature
- (2012)
- doi:10.1038/nature11617
- Received
- Accepted
- Published online
The prefrontal cortex (PFC) is thought to participate in high-level control of the generation of behaviours (including the decision to execute actions1); indeed, imaging and lesion studies in human beings have revealed that PFC dysfunction can lead to either impulsive states with increased tendency to initiate action2, or to amotivational states characterized by symptoms such as reduced activity, hopelessness and depressed mood3. Considering the opposite valence of these two phenotypes as well as the broad complexity of other tasks attributed to PFC, we sought to elucidate the PFC circuitry that favours effortful behavioural responses to challenging situations. Here we develop and use a quantitative method for the continuous assessment and control of active response to a behavioural challenge, synchronized with single-unit electrophysiology and optogenetics in freely moving rats. In recording from the medial PFC (mPFC), we observed that many neurons were not simply movement-related in their spike-firing patterns but instead were selectively modulated from moment to moment, according to the animal’s decision to act in a challenging situation. Surprisingly, we next found that direct activation of principal neurons in the mPFC had no detectable causal effect on this behaviour. We tested whether this behaviour could be causally mediated by only a subclass of mPFC cells defined by specific downstream wiring. Indeed, by leveraging optogenetic projection-targeting to control cells with specific efferent wiring patterns, we found that selective activation of those mPFC cells projecting to the brainstem dorsal raphe nucleus (DRN), a serotonergic nucleus implicated in major depressive disorder4, induced a profound, rapid and reversible effect on selection of the active behavioural state. These results may be of importance in understanding the neural circuitry underlying normal and pathological patterns of action selection and motivation in behaviour.




No comments:
Post a Comment